Abstract
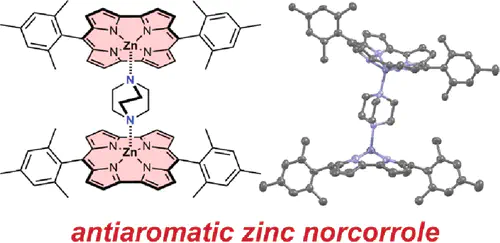
Zinc norcorrole was prepared as its pyridine complex (ZnNc·pyridine) by metalation of freebase norcorrole. The ZnNc·pyridine complex is distinctly bowl-shaped, as demonstrated by both X-ray crystallography and nuclear magnetic resonance (NMR) spectroscopy. NMR spectroscopy showed characteristic ring current deshielding effects, with different magnitudes on either face of the bowl-shaped complex. Exchanging the pyridine ligand with the bidentate ligand DABCO results in the formation of a stable (ZnNc)2·DABCO sandwich complex, which was also characterized by crystallography and NMR spectroscopy. The NMR resonances of the axial ligands in all of the complexes demonstrate that the paratropic ring current in zinc norcorrole is approximately 40 nA/T, which is comparable in magnitude to the diatropic ring current in porphyrin. Analysis of the ligand-exchange processes on addition of DABCO to ZnNc·pyridine showed that ZnNc coordinates to axial nitrogen-containing ligands with approximately 1000-fold higher binding constants than analogous zinc porphyrins.